Abstract
Introduction
The combination of rituximab & lenalidomide (R 2) is an established regimen for treatment of follicular lymphoma (FL), with efficacy reported in the first line and relapsed setting (Morchhauser NEJM 2018, Leonard JCO 2019). The inferior OS of patients who remain post-induction PET-CT positive (PET+ve) has also been demonstrated in both settings (Trotman Lancet Haem 2014, Lancet Oncol 2018, Ysebaert, ASH 2011). We sought to study the effect of R 2 in relapsed FL by examining its ability to convert to PET-negative (PET-ve) those patients who remain PET+ve after reinduction rituximab-chemotherapy.
Methods
This was a prospective, multicentre, Phase 2 study of patients with bulky Stage II, or Stage III-IV relapsed FL. Eligibility criteria were: at least stable disease on CT within 4-6 weeks of last cycle of re-induction rituximab-chemotherapy; ECOG ≤2; CrCL ³30mL/min; haemoglobin >80g/L, neutrophils >1.0 & platelets >75 x 10 9/L. Exclusion criteria were: histological transformation ≤12 months (mo); any interim-PET that was negative, and other malignancy ≤5 years. After enrolment pts underwent a centrally-reviewed PET within 8 weeks of D1 last cycle of re-induction rituximab-chemotherapy. Given the higher probability of further progression in the relapsed setting PET+ve was defined as a Deauville score (DS) 3-5. PET-ve patients were assigned rituximab maintenance q2mo for 2 years, and those remaining PET+ve were assigned R 2 to commence within 12 weeks. Lenalidomide schedule for R 2 pts was 10mg/d x 21 q28d, with dose modifications for tolerance, over a planned 2 years. Repeat PET scans were scheduled at 6 & 12 mo after starting R 2.
The primary endpoint was the rate of conversion from postinduction PET+ve to PET-ve in evaluable patients 6 mo after commencing lenalidomide. Evaluable patients were defined as those receiving >63 days of Lenalidomide. Sample size calculations used a one-sided exact test for proportions, assuming a conversion rate of ³50% as worthy of further evaluation and ≤20% as unacceptable. Thus 16 evaluable patients were required to have 80% power with type I error of 5%. Secondary endpoints were PET conversion rates by baseline DS in the PET+ve, the toxicity & deliverability of R 2, and PFS & OS in both the PET+ve & PET-ve populations.
Results
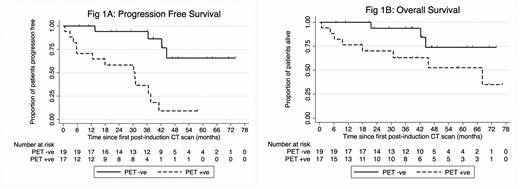
Thirty-seven patients (pts) were recruited from Nov 2013 to Jan 2021 when the study was closed due to poor recruitment attributed to competing studies. Median (med) age was 67yrs (36-83); 58% male, med 2 (range 2-11) prior therapies incl. the recent rituximab-chemotherapy; FLIPI low risk (21%), intermediate risk (12%) high risk (67%). Eighteen of 37 (48.6%) pts were postinduction PET+ve. Med follow-up was 38 mo (0.8 - 76.4): 32 mo (0.8 - 76.4) in PET+ve and 42 mo (6.7 - 73.8) in PET-neg. Of the 18 PET+ve pts one was ineligible for R 2 due to reactivation of hepatitis B; 3 were not evaluable having not received >63 doses of lenalidomide due to progressive disease (PD). Thus 14/18 (78%) PET+ve pts were evaluable, of whom 5/14 (36%; 95% CI 11% - 61%) became PET-ve at 6-mo, thus not excluding a PET conversion rate of <20%, (p=0.14). If we had obtained full recruitment both additional pts would have had to convert to PET-neg to meet the primary endpoint. PET conversion occurred in 4/6 evaluable pts with DS 3 and 1/8 with DS 5. PD occurred in 14 pts: 11/17 PET+ve and 3/19 PET-ve. Med PFS was 30.8 mo (5.7-37.6) in the PET+ve and NR (95% CI 42.3-NR) in the PET-ve, p = 0.0001. Death occurred in 11/37 (30%): 7 from lymphoma (5 PET+ve), 1 other malignancy, 2 pneumonia, 1 aspergillosis. Med OS was 68.1 mo (9.6 - NR) in PET+ve and NR (95% CI 42.3 - NR) in PET-ve (p 0.059).
Of the 17 PET+ve pts starting lenalidomide, deliverability was limited by both disease progression and AEs: 3 failed to receive 3 cycles, 6 pts received 4-6, and 8 pts 7-24 cycles. Mean number of lenalidomide doses was 213 (SD 188). At least one AE was reported in 16/17 (94%), most commonly neutropenia (n=10, 59%, Gd4 24%). At least one SAE occurred in 9/17 (53%): infections 2, malignancy 2, cardiac disorders 2, musculoskeletal 2, other causes 3 pts.
Conclusion
The high PET+ve rate of 49% (DS 3-5) after rituximab-chemotherapy for relapsed FL suggests the need for consolidation therapy. However, R 2 did not achieve a sufficiently high PET-conversion rate to justify further study. The inferior outcome of patients who remain PET+ve after treatment of relapse highlights the importance of investigating novel approaches in this setting.
Trotman: TAKEDA: Research Funding; beigene: Research Funding; roche: Research Funding; BMS: Research Funding; PCYC: Research Funding; JANSSEN: Research Funding. Gandhi: janssen: Research Funding; novartis: Honoraria. Butcher: WriteSource: Current Employment, Other: Medical writing for Pharma companies. Not pertinent to this abstract for which author is study Statisticiam.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal